Understanding Periodic Trends in Atomic Size
Related Articles: Understanding Periodic Trends in Atomic Size
Introduction
With great pleasure, we will explore the intriguing topic related to Understanding Periodic Trends in Atomic Size. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends in Atomic Size
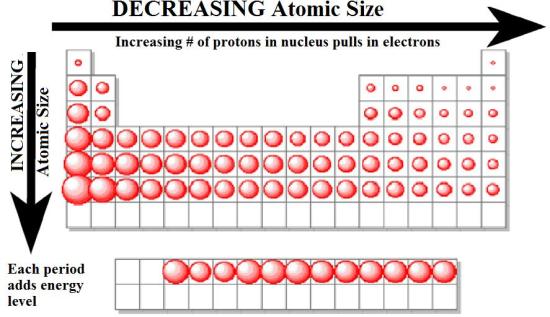
The periodic table is a fundamental tool in chemistry, organizing elements based on their properties and revealing predictable patterns. One of the most important trends observable is the variation in atomic size, which refers to the distance between the nucleus and the outermost electron shell of an atom. This seemingly simple concept holds significant implications for understanding the chemical behavior of elements and their interactions.
Atomic size is not a fixed value, but rather a complex concept that can be influenced by various factors. These include:
- Number of electron shells: As we move down a group (vertical column) in the periodic table, the number of electron shells increases. This leads to a larger atomic radius, as electrons are occupying orbitals further away from the nucleus.
- Effective nuclear charge: The effective nuclear charge is the net positive charge experienced by an electron in an atom. It is influenced by the number of protons in the nucleus and the shielding effect of inner electrons. As we move across a period (horizontal row) in the periodic table, the effective nuclear charge increases, pulling the electrons closer to the nucleus and resulting in a smaller atomic radius.
- Shielding effect: Inner electrons shield the outer electrons from the full attraction of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outer electrons, leading to a larger atomic radius.
- Electron configuration: The arrangement of electrons in orbitals can also influence atomic size. For example, atoms with a half-filled or completely filled subshells exhibit greater stability and a slightly smaller atomic radius due to increased electron-electron repulsion.
Factors Affecting Atomic Size
1. Number of Electron Shells:
The number of electron shells directly influences atomic size. As we move down a group, elements gain additional electron shells, resulting in a larger atomic radius. This is because the outermost electrons are further away from the nucleus, leading to a greater spatial extent. For instance, lithium (Li) has a single electron shell, while sodium (Na) has two, and potassium (K) has three. Consequently, potassium has the largest atomic radius among these three elements.
2. Effective Nuclear Charge:
The effective nuclear charge is the net positive charge experienced by an electron in an atom. It is calculated by subtracting the number of core electrons (those in inner shells) from the number of protons in the nucleus. As we move across a period, the number of protons increases, resulting in a higher effective nuclear charge. This stronger attraction pulls the electrons closer to the nucleus, leading to a smaller atomic radius. For example, lithium (Li) has a smaller atomic radius than beryllium (Be) because beryllium has a higher effective nuclear charge.
3. Shielding Effect:
The shielding effect arises from the repulsion between inner electrons and outer electrons. Inner electrons shield the outer electrons from the full attraction of the nucleus, reducing the effective nuclear charge experienced by the outer electrons. This results in a larger atomic radius. The extent of shielding depends on the number of inner electrons and their orbital shapes. For example, the shielding effect is greater in elements with more inner electrons, leading to a larger atomic radius.
4. Electron Configuration:
The arrangement of electrons in orbitals can also affect atomic size. Elements with a half-filled or completely filled subshells exhibit greater stability due to increased electron-electron repulsion. This increased repulsion can lead to a slightly smaller atomic radius. For example, nitrogen (N) has a smaller atomic radius than oxygen (O) due to the half-filled p-orbitals in nitrogen.
Trends in Atomic Size
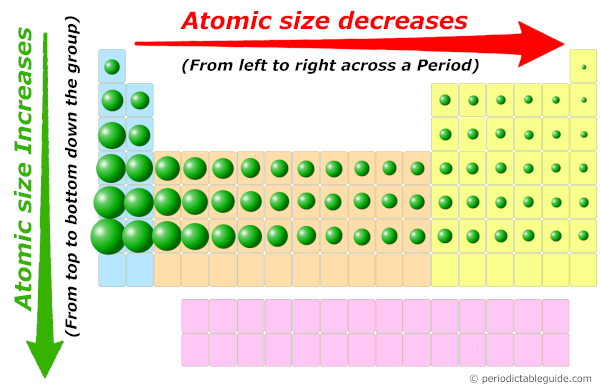
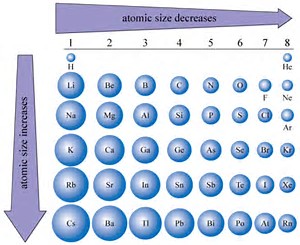
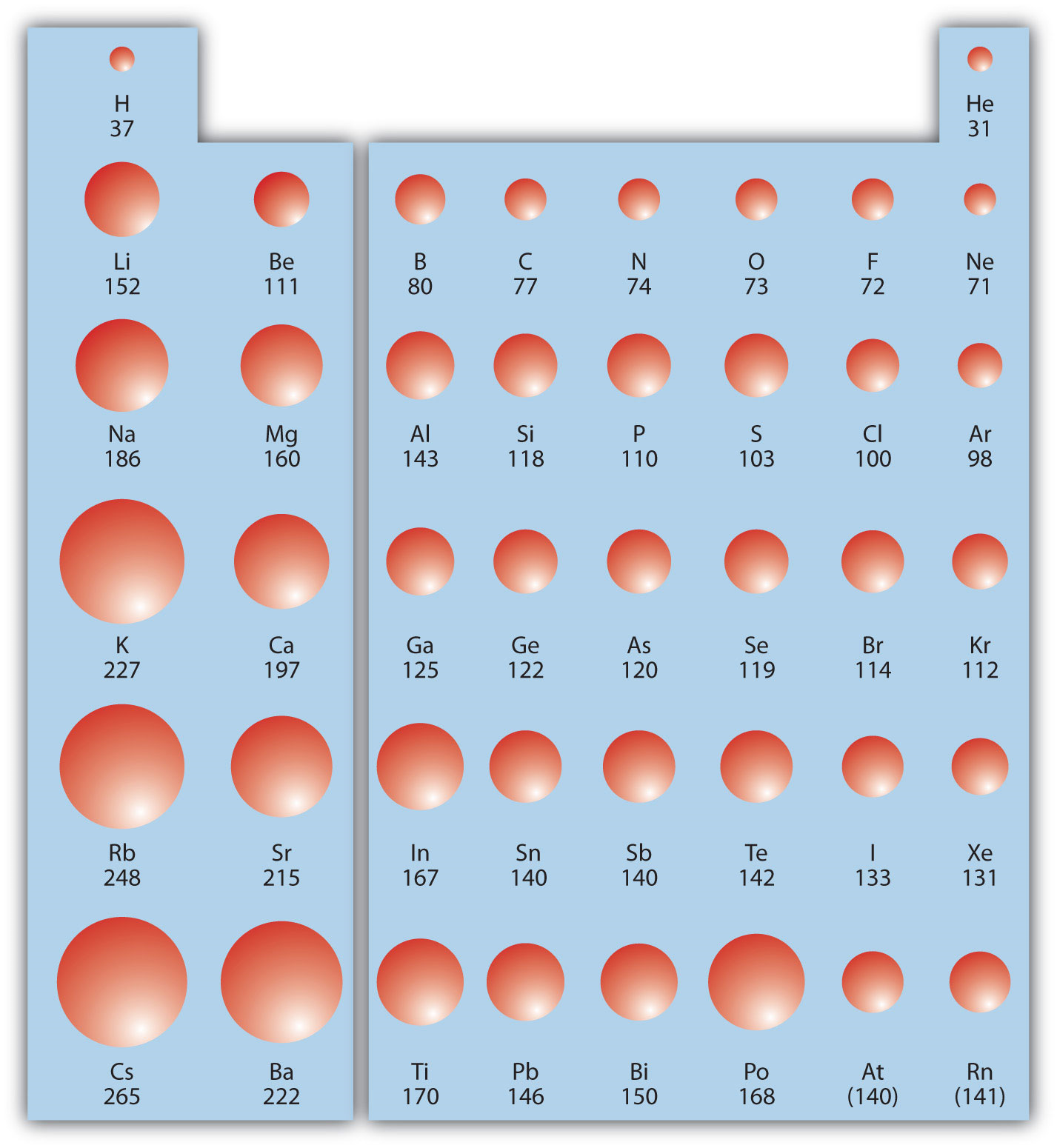
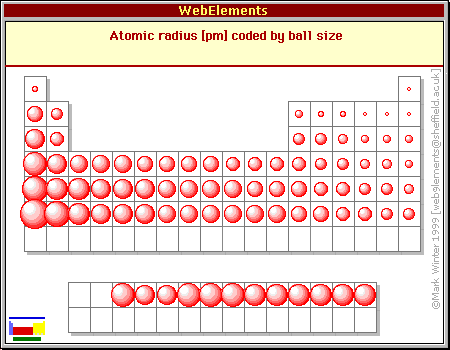
1. Atomic Size Increases Down a Group:
As we move down a group, the number of electron shells increases, leading to a larger atomic radius. This is because the outermost electrons are further away from the nucleus. For example, lithium (Li) has a smaller atomic radius than sodium (Na) and potassium (K), which have more electron shells.
2. Atomic Size Decreases Across a Period:
As we move across a period, the number of protons increases, resulting in a higher effective nuclear charge. This stronger attraction pulls the electrons closer to the nucleus, leading to a smaller atomic radius. For example, lithium (Li) has a larger atomic radius than beryllium (Be) and boron (B), which have higher effective nuclear charges.
3. Anomalies in Atomic Size:
There are some exceptions to the general trends in atomic size. For example, the atomic radius of elements in the second period is slightly larger than expected due to the smaller size of the 2s orbital compared to the 3s orbital. Additionally, elements with half-filled or completely filled subshells can exhibit slightly smaller atomic radii due to increased electron-electron repulsion.
Importance of Understanding Atomic Size
1. Chemical Reactivity:
Atomic size plays a crucial role in determining the chemical reactivity of elements. Elements with larger atomic radii tend to be more reactive because their outermost electrons are further away from the nucleus and are more easily removed. For example, alkali metals (Group 1) have large atomic radii and are highly reactive.
2. Bonding Properties:
The size of atoms influences the types of bonds they can form. Smaller atoms tend to form stronger covalent bonds due to the increased overlap of their atomic orbitals. Larger atoms, on the other hand, are more likely to form ionic bonds, where electrons are transferred from one atom to another.
3. Physical Properties:
Atomic size influences various physical properties of elements, such as melting point, boiling point, and density. Elements with larger atomic radii tend to have lower melting and boiling points due to weaker interatomic forces.
4. Biological Significance:
Atomic size is crucial for understanding the structure and function of biological molecules. For example, the size of atoms plays a role in the binding of enzymes to substrates and the formation of DNA and RNA.
Related Searches
1. Atomic Radius vs. Ionic Radius:
While atomic radius refers to the size of a neutral atom, ionic radius refers to the size of an ion. Ions can be either cations (positively charged) or anions (negatively charged). Cations are smaller than their corresponding neutral atoms because they have lost electrons, reducing the electron-electron repulsion and allowing the remaining electrons to be pulled closer to the nucleus. Anions, on the other hand, are larger than their corresponding neutral atoms because they have gained electrons, increasing the electron-electron repulsion and pushing the electrons further away from the nucleus.
2. Periodic Trends in Ionization Energy:
Ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground state. It is a measure of the strength of the attraction between the nucleus and the outermost electron. As we move down a group, ionization energy decreases because the outermost electron is further away from the nucleus and is more easily removed. As we move across a period, ionization energy increases because the effective nuclear charge increases, making it more difficult to remove an electron.
3. Periodic Trends in Electron Affinity:
Electron affinity is the change in energy when an electron is added to a neutral gaseous atom to form a negative ion. It is a measure of the atom’s ability to gain an electron. As we move down a group, electron affinity generally decreases because the added electron is further away from the nucleus and experiences less attraction. As we move across a period, electron affinity generally increases because the added electron experiences a stronger attraction to the nucleus.
4. Periodic Trends in Electronegativity:
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. It is influenced by both the effective nuclear charge and the distance of the valence electrons from the nucleus. As we move down a group, electronegativity decreases because the valence electrons are further away from the nucleus and experience a weaker attraction. As we move across a period, electronegativity increases because the effective nuclear charge increases, making the atom more attractive to electrons.
5. Periodic Trends in Melting and Boiling Points:
Melting point and boiling point are physical properties that are influenced by the strength of interatomic forces. Elements with larger atomic radii tend to have weaker interatomic forces due to the greater distance between atoms. This results in lower melting and boiling points. For example, alkali metals (Group 1) have large atomic radii and low melting and boiling points.
6. Periodic Trends in Density:
Density is a measure of mass per unit volume. Elements with larger atomic radii tend to have lower densities because they have a larger volume for a given mass. For example, alkali metals (Group 1) have large atomic radii and low densities.
7. Periodic Trends in Reactivity:
The reactivity of an element is related to its ability to gain or lose electrons. Elements with larger atomic radii tend to be more reactive because their outermost electrons are further away from the nucleus and are more easily removed. For example, alkali metals (Group 1) have large atomic radii and are highly reactive.
8. Periodic Trends in Metallic Character:
Metallic character refers to the tendency of an element to lose electrons and form positive ions. Elements with larger atomic radii tend to have a greater metallic character because their outermost electrons are further away from the nucleus and are more easily removed. For example, alkali metals (Group 1) have large atomic radii and exhibit strong metallic character.
FAQs
Q: What is the difference between atomic radius and ionic radius?
A: Atomic radius refers to the size of a neutral atom, while ionic radius refers to the size of an ion. Cations are smaller than their corresponding neutral atoms, while anions are larger.
Q: How does atomic size affect chemical reactivity?
A: Elements with larger atomic radii tend to be more reactive because their outermost electrons are further away from the nucleus and are more easily removed.
Q: What are some exceptions to the general trends in atomic size?
A: There are some exceptions to the general trends in atomic size, such as the larger atomic radius of elements in the second period compared to expected and the slightly smaller atomic radii of elements with half-filled or completely filled subshells.
Q: How does atomic size influence the physical properties of elements?
A: Atomic size influences various physical properties, such as melting point, boiling point, and density. Elements with larger atomic radii tend to have lower melting and boiling points due to weaker interatomic forces.
Q: What is the biological significance of atomic size?
A: Atomic size is crucial for understanding the structure and function of biological molecules, such as the binding of enzymes to substrates and the formation of DNA and RNA.
Tips
- Visualize the periodic table: Use a periodic table as a visual aid to understand the trends in atomic size.
- Focus on the key factors: Remember the four main factors that influence atomic size: number of electron shells, effective nuclear charge, shielding effect, and electron configuration.
- Practice with examples: Use examples of elements to illustrate the trends in atomic size.
- Connect the trends to other properties: Understand how atomic size relates to other periodic trends, such as ionization energy, electron affinity, and electronegativity.
- Consider the exceptions: Be aware of the exceptions to the general trends in atomic size.
Conclusion
Understanding periodic trends in atomic size is essential for comprehending the chemical behavior of elements and their interactions. The size of an atom is influenced by a complex interplay of factors, including the number of electron shells, effective nuclear charge, shielding effect, and electron configuration. By recognizing these trends, we gain a deeper understanding of the chemical properties of elements and their role in various chemical reactions and biological processes.
The periodic table is a powerful tool for organizing and understanding the properties of elements. By studying periodic trends, we can predict the behavior of elements and design new materials with specific properties. The knowledge of atomic size and other periodic trends is crucial for chemists, biologists, and other scientists in their endeavors to unravel the mysteries of the natural world.

/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)

Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends in Atomic Size. We thank you for taking the time to read this article. See you in our next article!